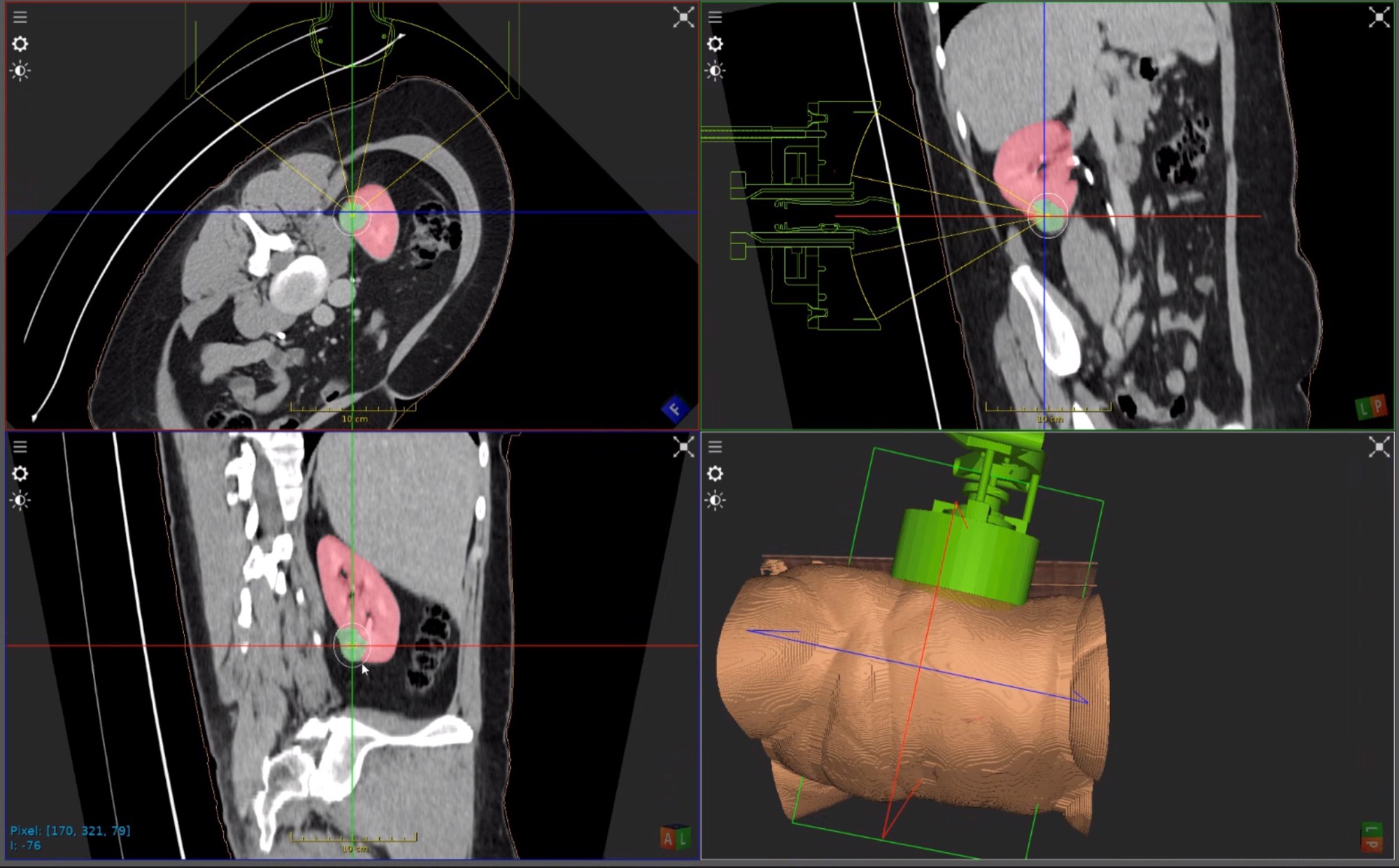
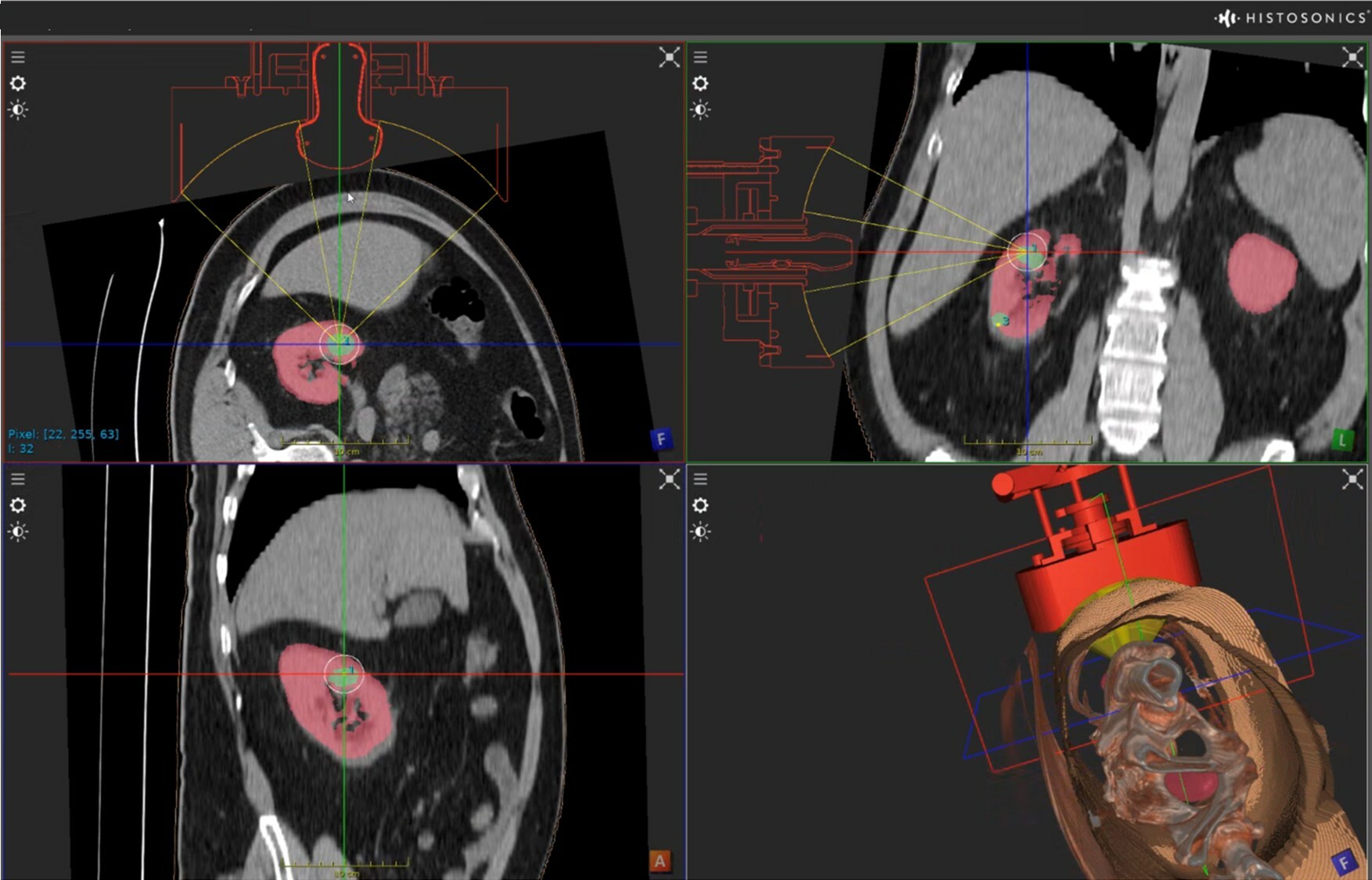
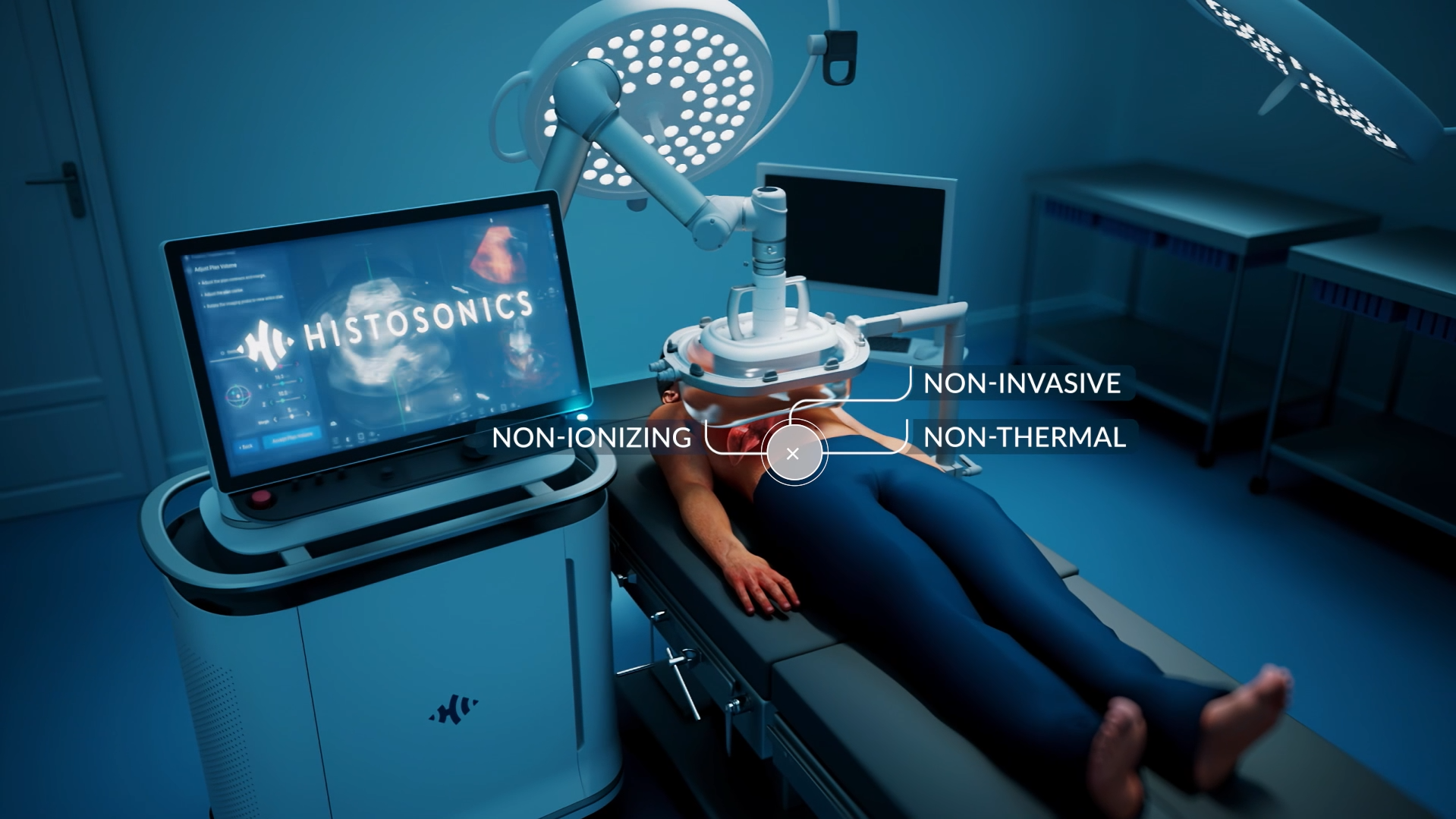
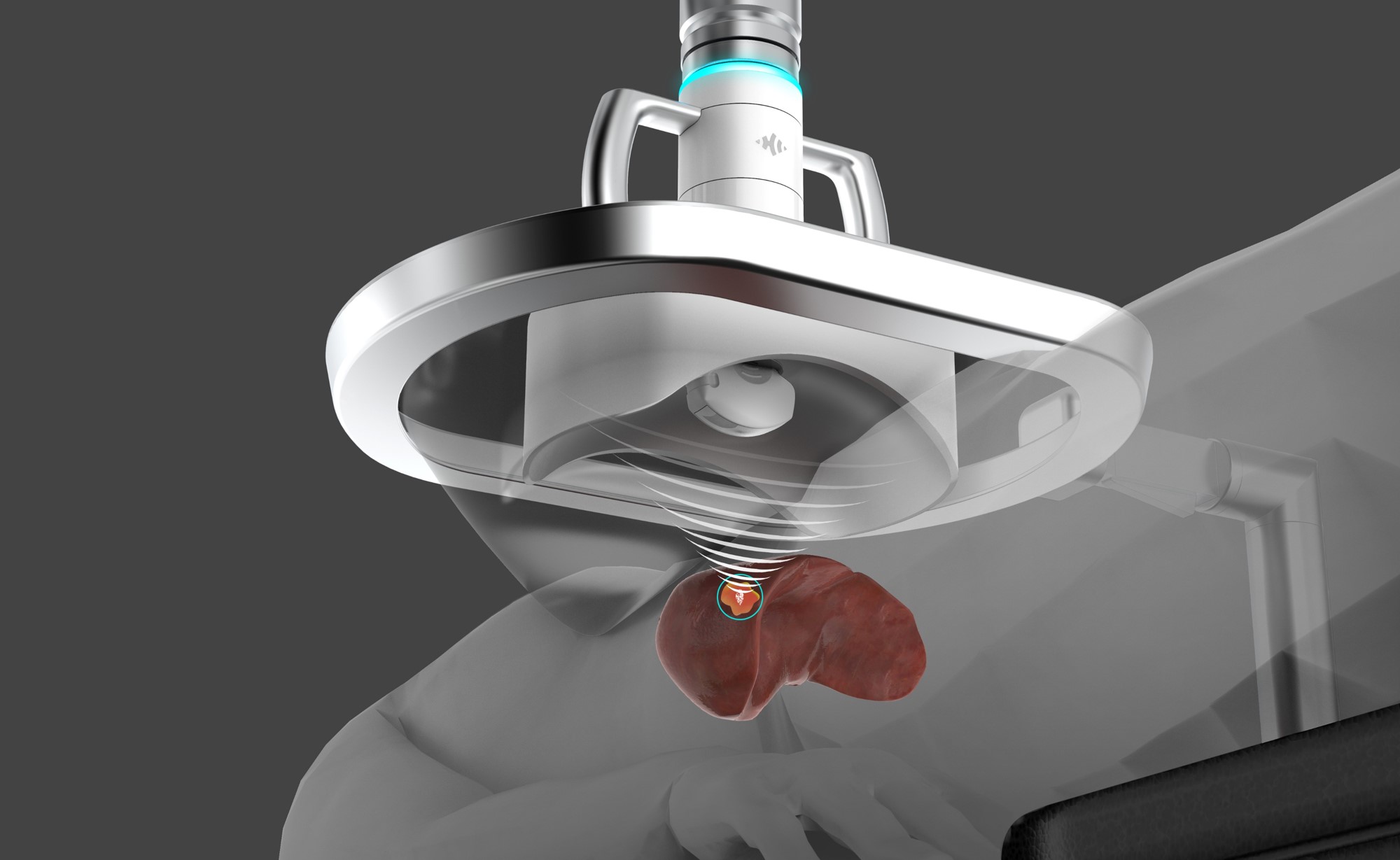
HistoSonics Awarded Key Position in UK’s Novel Innovation Program
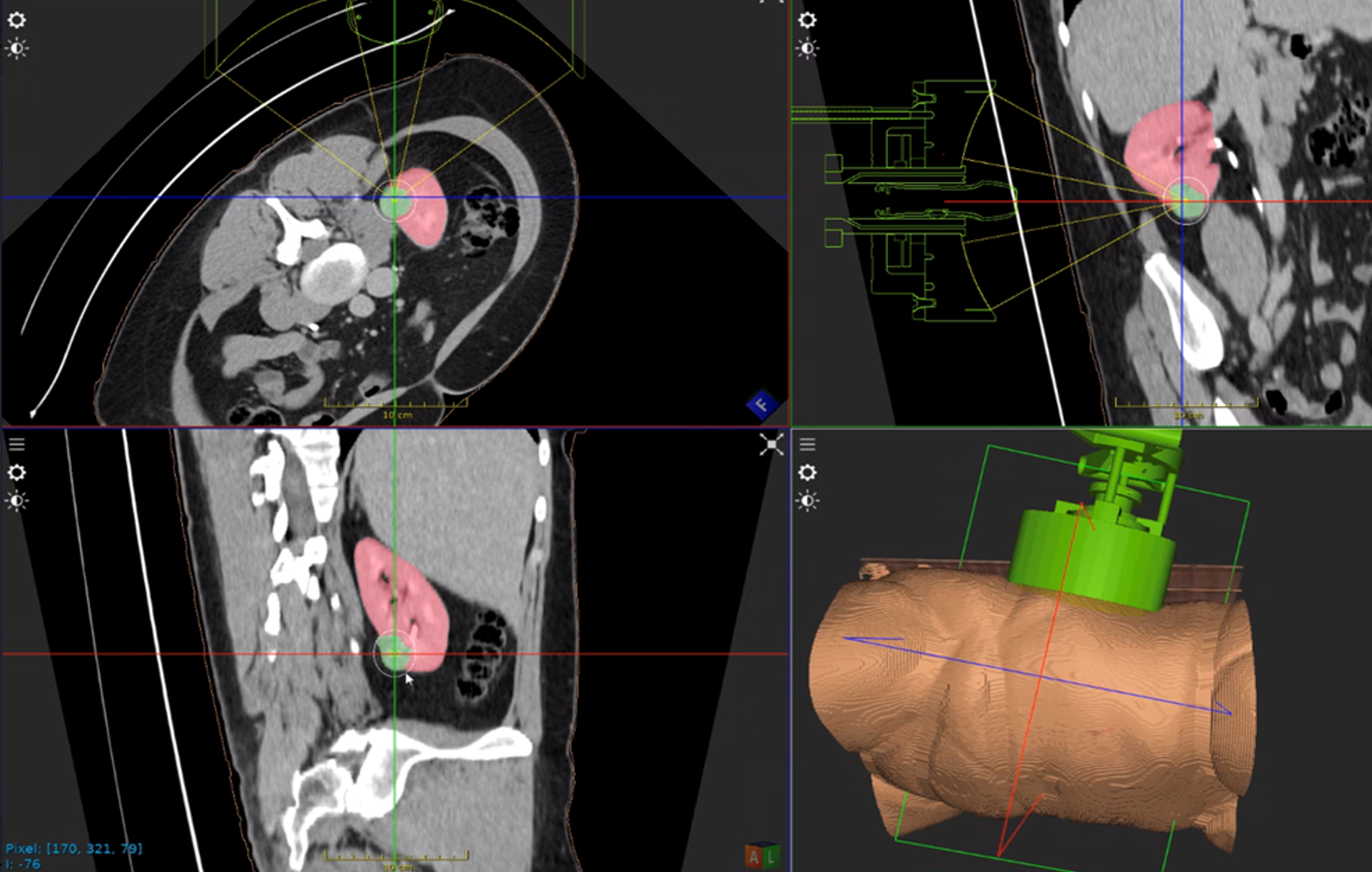
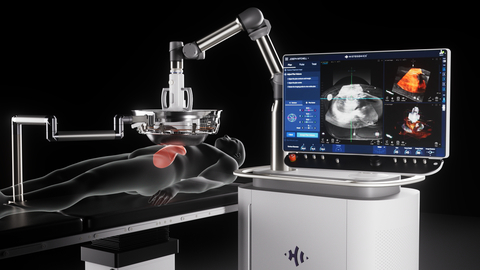
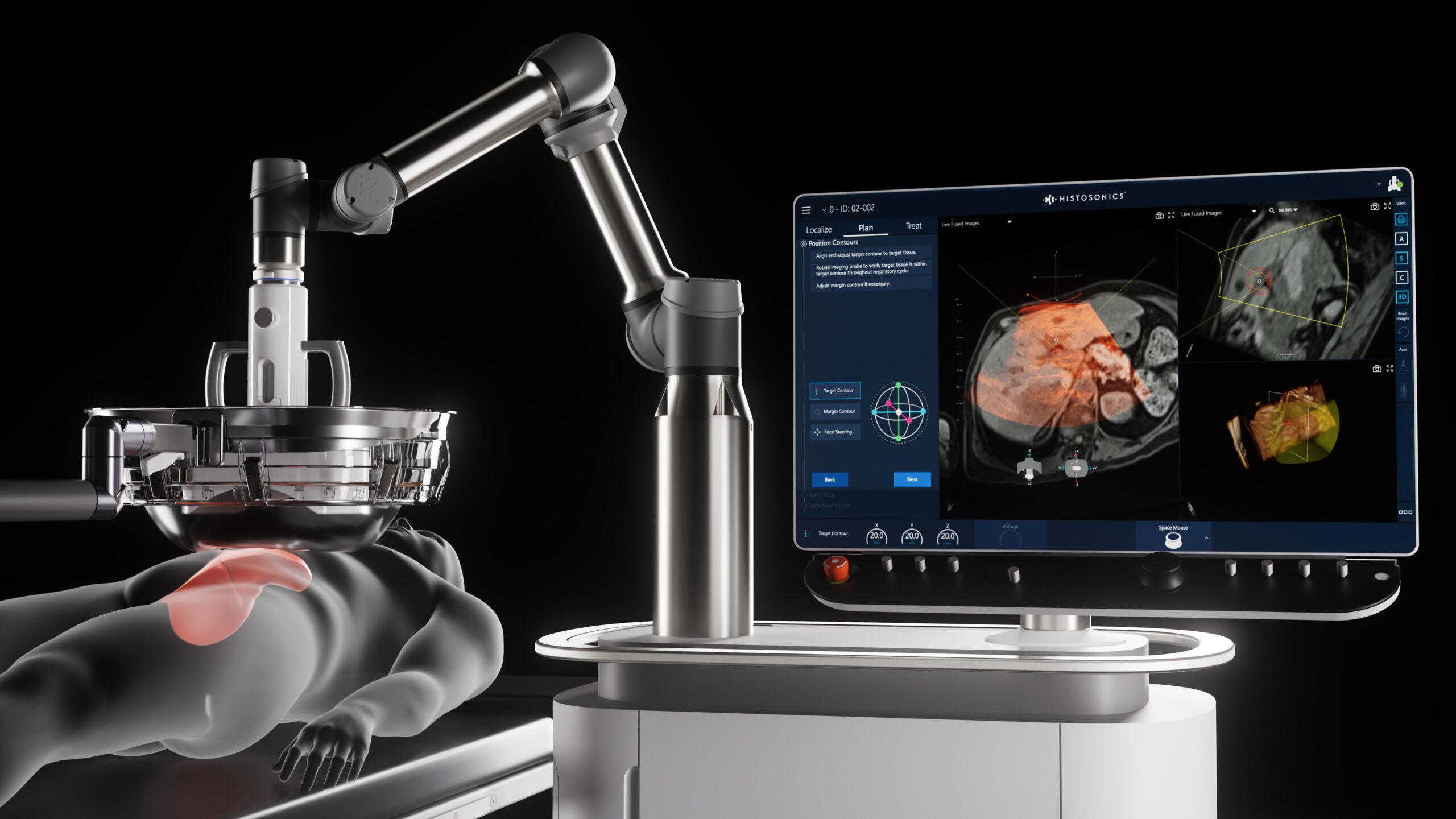
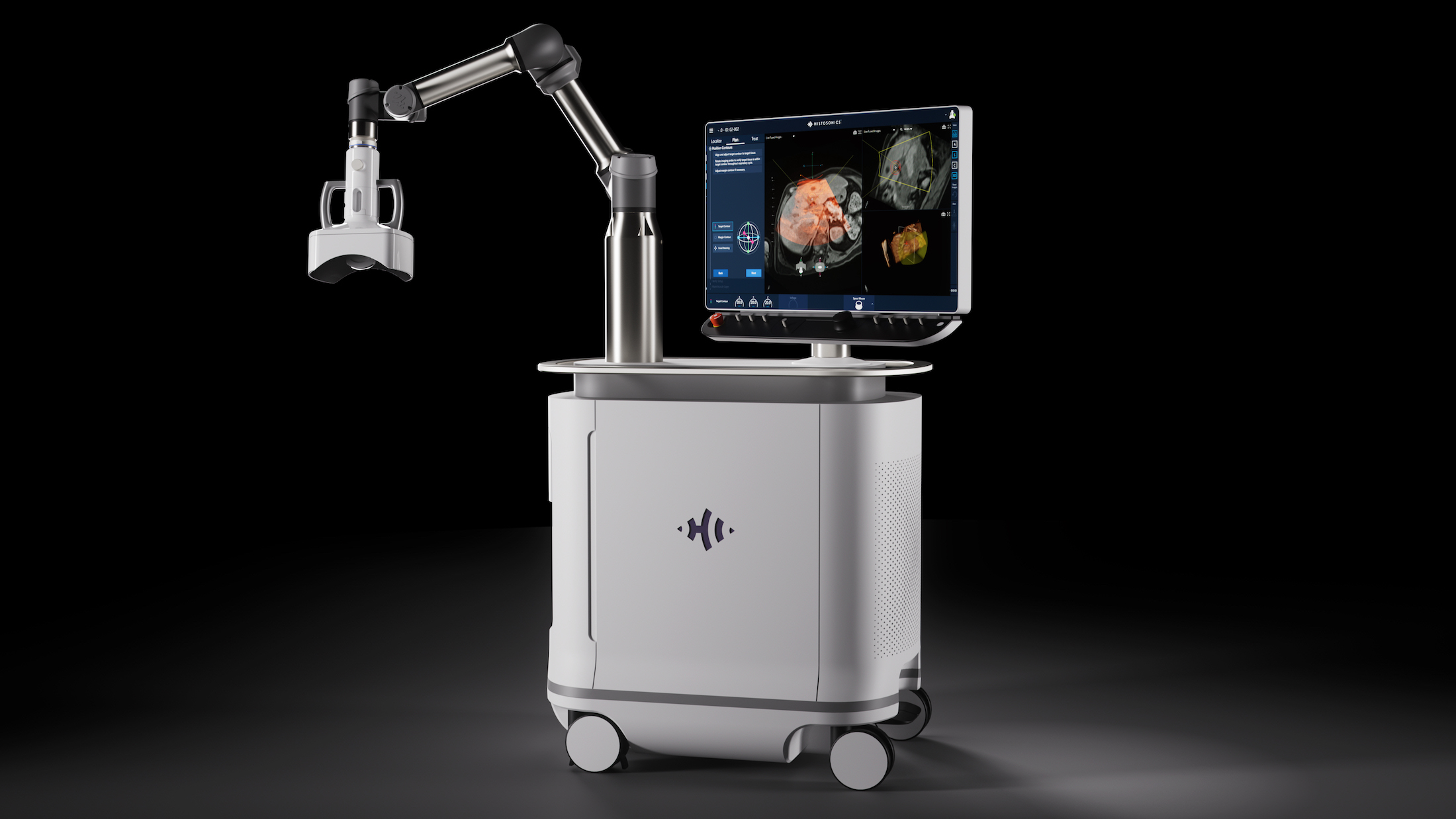
Edison Histotripsy System Selected for Competitive Accelerated Market Access Program Minneapolis, MN. February 14th , 2024 – HistoSonics ® , (www.histosonics.com), the manufacturer of the Edison ® System and novel histotripsy therapy platforms, announced today that the company’s Edison System has been selected to participate in the UK’s newly created Innovative Devices Access Pathway (IDAP) […]